Glass Strength
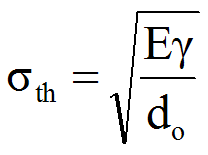
Glass is not like other products used in buildings. It is clear, fairly rigid providing a lot of strength but can be brittle as well. At an atomic level glass is a network of silicon and oxygen bonds modified randomly by sodium. The structure is not regular as it would be if it were liquid. The network of bonds prevent the glass from being ductile. The ingredients for glass are not transparent and it is the melting of these products that creates new bonds. As the glass cools the ingredients are locked together before they can resume their original structures.
The notion that glass is a supercooled liquid is a misnomer. Glass during manufacture is cooled to a solid state. For many materials the atoms return to their original state when cooled but for glass the backward transition is restricted and the new structure maintained. The rigidity of the product sets it apart from other materials. Most sheet products will deform when placed under load. The structure allows atoms or molecules to move past each other where glass does not. The freedom in other materials may lead to permanent deformation where glass returns to its shape once the load is removed. Forces applied to materials give rise to stress which is a measure of the internal forces per unit area. Not surprisingly the relationship between stress and strain in glass is linear because of its inability to behave in a plastic way.
When glass is under load it will bend and accommodate stress to a certain level and then suddenly fail once its threshold is met. The failure can be sudden and spectacular. Once a crack starts there is little within its structure to stop it propagating. A more positive point is that because the inner structure is not mobile glass does not suffer from dynamic fatigue. Once stress is removed the glass returns unchanged by the experience. A plate of glass in a window exposed to wind over years does not fail because it is constantly being stressed by the wind.
The strength of glass is determined by putting the glass under stress until it breaks. The surface strength is measured using a ring and the edge strength by 4 point bending. The test is repeated to derive a distribution for the break strength. Even with glass taken from the same plate it cannot be assumed that the glass will break at exactly the same load. The distribution of the range of test results is expressed by the Weibull modulus. From this we know how predictable the glass strength is and how strong the weakest sample was. We then have data for the glass properties that we can use to compare both stress and deflection for a given load.
There are processes that increase the strength of glass. Toughening glass takes the glass to a temperature where it becomes flexible again and then it is chilled evenly and rapidly. The outer surface is chilled quicker than the inner glass. Because the core is trying to contract and the outer surfaces are already chilled the outer surface is being compressed while the core is under tension. The compression at the surface is pulling the glass together so any flaws are being closed rather than being free to open. It is only when the compressive strength is overcome that the glass will fail. The energy stored in the glass is released and the glass breaks in the characteristic form of toughened glass. Toughened glass is predictably 5 times stronger that annealed glass of the same thickness.